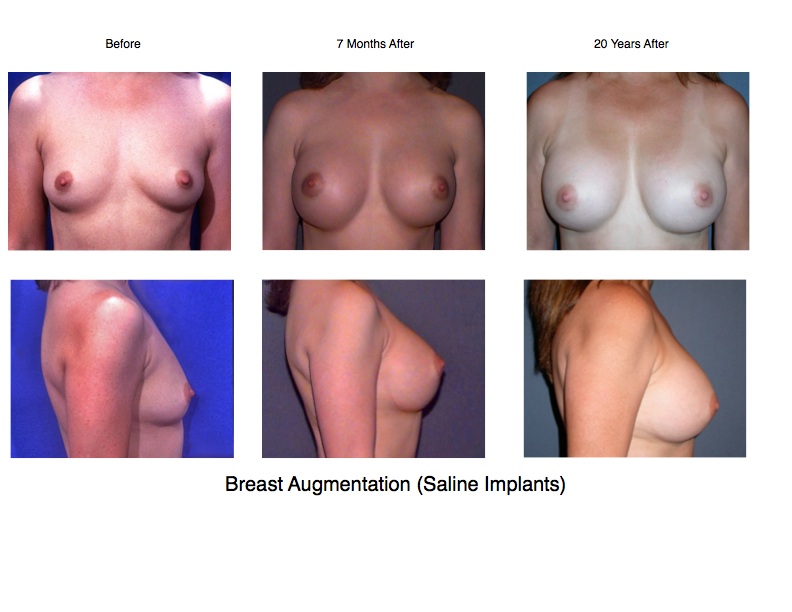
Patients considering breast augmentation should take the time to read this carefully!
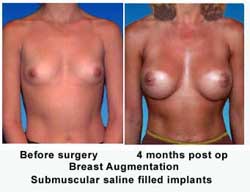
Breast augmentation has been one of the most gratifying cosmetic operations from the standpoint of both the patient and the surgeon. Although controversy in the early 1990’s led to widespread fear about the possibility of silicone breast implants causing autoimmune diseases, a significant body of recent scientific research has validated the overall general safety of silicone breast implants. Although every operation involves some risks, breast augmentation is generally not considered to be dangerous. The operation is carried out in our Cosmetic Surgical Center under anesthesia on an outpatient basis. It generally takes about an hour.
While breast augmentation will enlarge the breasts, it will not alter basic defects in breast shape or form. Major asymmetries may be improved, but will not be eliminated. Slight differences in the size or shape of the two breasts are considered normal and should not be a cause for concern. Long experience with this operation has demonstrated highly satisfactory results for the majority of patients who are considered suitable candidates for the surgery.
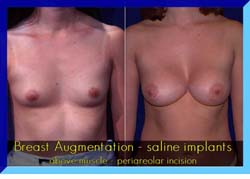
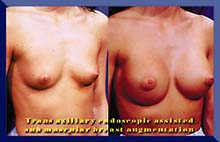
CURRENT STATUS OF BREAST IMPLANTS
Modern silicone prostheses have been used for more than thirty years. Both silicone gel and saline filled implants have been used. Extensive studies have been carried out and there has been no evidence that the prostheses have any relationship to breast cancer, autoimmune disease or any other systemic illnesses in patients. Specifically, patients with breast implants have no higher incidence of autoimmune or connective tissue disease such as rheumatoid arthritis, scleroderma or lupus, in comparison with the general population.
The Food & Drug Administration has approved the use of saline filled silicone implants for both cosmetic and reconstructive surgery. The use of gel filled silicone implants has also been recently approved with certain restrictions which are currently evolving.
Prospective patients must make their own decision as to whether they wish to take the risks involved in the operation. As of this writing, we are continuing to recommend saline filled implants, but we are certainly happy to use of gel if patients request.
Our experience with saline filled implants has convinced us that results are generally equal to those that were formerly achieved with gel filled implants. Recognizing that there is a high degree of safety with gel filled implants, one cannot avoid the fact that there is some added risk if leakage occurs. While unlikely, it is possible for the gel to diffuse into surrounding tissues causing inflammatory reactions, lymph node enlargement or nerve injury. This risk is avoided with saline filled implants, since the salt water that is used to fill the device will be quickly absorbed by the body if there is any leakage.
BREAST EXAMINATION AFTER SURGERY
Following augmentation, careful routine examination for breast changes remains very important. Some studies have suggested that it may be more difficult to detect early breast cancer on routine mammography, although this has not been proven and other authorities feel that with proper technique, mammography can be as successful in augmented patients as in the normal population. Special techniques should allow patients to detect cancer without significant increased risk according to most present information, as long as the implants remain soft.
DURABILITY OF BREAST IMPLANTS
Based on current experience, the prosthesis should last for many years. However, since no breast prostheses have been implanted for a full life span, it is impossible to give an unequivocal statement in this regard. Our own experience suggests to us that there is approximately an annual 1% risk of leakage from saline implants. It is important to emphasize that this is an estimate, and future data may lead to a better knowledge of the overall long-term risk of leakage.
Although leakage is relatively infrequent, it is an ongoing problem that can occur at any stage following surgery. If leakage occurs with a saline implant, replacement is generally quite simple with low risk and little discomfort. Patients normally can return to normal activity within a day or so of the replacement procedure. The issue is different with gel implants since leakage is generally not readily apparent.
Leakage from a gel implant can be very difficult to detect and often will not show up on a mammogram. MRI examination or direct endoscopy are the only ways to detect early leakage in a gel implant. For this reason, the FDA has recommended bi-annual MRI examinations for patients having gel implants. Since patients almost never follow this recommendation, most surgeons recommend routine replacement of the implants after 10 years. We agree with this recommendation. It is extremely important for patients to report any signs of leakage as soon as possible. If the implant begins to deflate, the scar tissue surrounding the implant will quickly begin to shrink. If this has occurred, when the implant is finally replaced the pocket will be overly tight resulting in an unnatural firmness. When this occurs, further corrective surgery is required and can result in the need for additional surgery after replacement, or a more extensive operation at the time of replacement.
RISKS
As is the case in all surgery, there are certain risks that are inherent in this operation. Although the scars are usually well hidden, irregularity or thickening of scars can occur which might require revision. Rarely, hemorrhage may require removal of prosthesis to control the bleeding. Infection is probably the most serious risk of breast augmentation. If an infection occurs, antibiotics alone will rarely clear up the infection unless the implant is removed. It is necessary to leave the implant out for a period of about three months before it is safe to attempt replacement. The risk of infection during the first year as reported by the manufacturers is between .5 and 1%. Our personal experience indicates a risk of about .2%. While it is usually confined to the early post op period, infection from mycobacteria can occur much later. Fortunately the prosthesis can usually be successfully replaced at a later time after infection has completely resolved.
Another potentially serious complication is implant rejection. Silicone is the least reactive material used for implant construction. Most patients tolerate the material without difficulty. Nevertheless, a very small percentage will react to the material making successful augmentation an impossibility. Fortunately, this is a very uncommon problem.
Sensory changes can occur resulting in numbness or discomfort, and while these symptoms are usually not long-standing or severe, they can be in some cases. Implants could possibly interfere with nursing although many patients nurse after the operation without difficulty. No studies to date have indicated that this is likely.
FIRMNESS AFTER AUGMENTATION
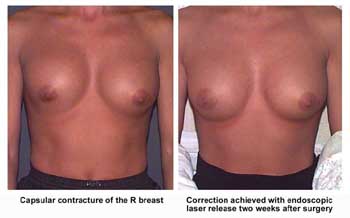
One problem that can occur with breast implants is related to the natural tissue capsule that forms around the implant within the body. Sometimes this capsule thickens or contracts causing unnatural firmness or shape to the breast. In severe cases, it can also cause pain or discomfort and can lead to the formation of fine calcium particles. Saline implants appear to be less likely to result in firmness than gel filled implants. This condition is called “capsular contracture”
In early cases, some surgeons believe that this might be improved by compression exercises, although we do not share this viewpoint. In more advanced cases, further surgery is required.
For years, many surgeons have believed that by applying a textured surface to the silicone implant, ingrowth of tissue to that surface would decrease the risk of firmness. This belief was based on experience years ago with polyurethane covered implants. These implants were found to remain soft in the vast majority of patients. Some studies in the medical literature confirmed that idea that texturing reduced the risk of firm encapsulation.
At our Center, we had long been advocates of textured implants. Now, based on recent evidence presented to the FDA by the implant manufacturers, we re-evaluated that position. The data failed to indicate any benefit from the use of textured implants. And our own experience failed to demonstrate any benefit of surface texturing.
Our experience suggests an average 1% annual risk of patients developing capsular contracture severe enough to indicate surgical revision. However, patients should be aware that the studies presented to the FDA indicate that the overall risk of requiring further surgery is significantly higher and could be as high as 15 to 20% within the first three years following surgery.
In the past, surgeons often recommended firm compression to treat capsular contracture; a procedure called closed capsulotomy. This is no longer recommended, because of the risk of breaking the outer membrane of the implant. Currently, we are using a laser for this type of treatment as part of a relatively simple endoscopic operation that releases the scar tissue. Unfortunately, there is no certainty that the scar tissue may not reform. In more advanced cases of capsular contracture, open surgery is required for correction.
Laser endoscopic capsulotomy can often replace a more extensive and invasive procedure. The operation is carried out under general anesthesia and involves a very small incision beneath the nipple. A small endoscope, or telescope, is inserted into the wound between the fibrous capsule and the actual implant. Attached to the telescope is a camera and a laser which is used to cut open the scar tissue and release the contracture. After surgery, patients generally notice an immediate softening of the breast. Recovery is prompt with no special precautions, allowing patients to resume normal activity the next day. The technique works best if carried out before the scar tissue is too thick, so patients are wise to consider treatment before the condition progresses too far.
RIPPLING
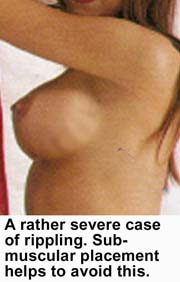 Rippling, or surface irregularities over the implant, is a potential problem with any type of breast implant. It occurs somewhat more frequently with saline filled implants because the fluid in the implant is less viscous than the silicone gel. The added risk of rippling is the trade-off for the increased safety of the saline filled device. Because of this potential problem, many patients favor submuscular placement of the implant. This is because it affords more soft tissue coverage over the upper portion of the implant.
Rippling, or surface irregularities over the implant, is a potential problem with any type of breast implant. It occurs somewhat more frequently with saline filled implants because the fluid in the implant is less viscous than the silicone gel. The added risk of rippling is the trade-off for the increased safety of the saline filled device. Because of this potential problem, many patients favor submuscular placement of the implant. This is because it affords more soft tissue coverage over the upper portion of the implant.
Rarely can rippling be seen or felt through the muscle, but unfortunately, the muscle does not cover the entire implant. Since the muscle only covers the upper portion of the implant rippling can still occur where there is inadequate coverage. The bulk and size of the pectoralis muscle varies from individual to individual, and so there is no set rule as to how much of the implant will be protected. A definite factor influencing the possibility of rippling is implant size. Larger implants tend to stretch and thin out the overlying tissue resulting in less natural coverage over the implant. For that reason, rippling is more common in patients who select larger implant sizes. This is a factor to be taken into consideration when selecting implant size.
Our experience has shown that while a significant percentage of patients may be aware of some rippling, relatively few find it of sufficient magnitude to be objectionable. If it is a problem, a possible treatment consists of overfilling of the implant. This is a surgical procedure in which added saline is placed inside the implant. By overfilling, the ripples are smoothed out to a certain degree by the added pressure of the fluid placed within the implant. We have had experience with a small number of patients who have elected to have this secondary procedure. The result of the operation is a much firmer implant but, so far, our patients have generally been pleased with the result. Another option is to reinject the patient’s own fat over the rippled area and, finally, patients may choose to replace their implants with gel.
IMPLANT SIZE
In selecting the size of the implant, the general choice should be jointly made by the patient and the surgeon prior to surgery. While ultimately, the choice of size is made by the patient, she should recognize that there are advantages to making a conservative selection. Both capsular contracture and rippling are more common with larger implants because of the added stretching.
There are several ways in which the patient can help to indicate the desired size. We recommend that our patients purchase a soft unpadded bra in the approximate cup size that they believe they would like to achieve. By using Baggies filled with bird seed or rice to estimate the approximate additional volume that they desire, patients can estimate the size implant that would be required to help them achieve their desired result. The final decision, of course, will be made during the time of surgery, based upon the patient’s desires, as well as which implant seems to fit and look best. In some cases, this could result in the breast being augmented slightly more or less than the patient had anticipated.
 ABOVE OR BELOW THE MUSCLE
ABOVE OR BELOW THE MUSCLE
The breast normally covers a muscle on the chest wall called the pectoralis muscle. Breast implants can be placed above or below this muscle. When implants are placed below the muscle, it is called a submuscular placement or a subpectoral placement. When the implant is placed above the muscle, it is called a subglandular or submammary placement, meaning that it’s below the mammary gland.
We have discussed the problem of rippling following breast augmentation in women with little natural soft tissue padding. This is often the case in women who have nursed children and lost breast substance or firmness. An excellent way to minimize this problem is to place the implants beneath the muscle. While this technique adds to the postoperative pain, it does not lead to any decrease in muscle function. At our Center, submuscular augmentation is preferred by the majority of our patients.
Another possible advantage of submuscular placement is that it allows for better mammography. It is generally felt that there is less chance of missing a lesion on mammography when the implant is below the muscle. The pectoralis muscle tends to hold the implant against the chest wall during mammography. Another advantage of submuscular placement is that the implant is entirely beneath the breast tissue, decreasing the possibility of interference with breast function.
It is also widely felt that when implants are put beneath the muscle, there is less likelihood of firmness developing. Perhaps this is the result of the pressure of the muscle stretching the scar tissue around the implant. We have not personally observed this to be the case.
A minor disadvantage of submuscular placement is that it results in a more painful recovery than the subglandular approach. Another disadvantage is that there can be some movement of the implant when forcibly flexing the pectoralis muscle.
 The major disadvantage of the submuscular approach is the possibility of incomplete settling. When placement is submuscular, the force of the muscle can shift the position of the implant before there is enough healing to hold it in place. For this reason, submuscular implants may seem abnormally high for a period of time, although eventually they usually settle to a desirable position. If they don’t (and this occurs in about 3 to 5% of patients with submuscular placement), an endoscopic procedure similar to that used for treatment of capsular contracture is effective at allowing proper positioning of the implant. While this procedure is almost alway successful, it does represent a possible added expense. For that reason, we are seeing more and more patients choose the subglandular approach.
The major disadvantage of the submuscular approach is the possibility of incomplete settling. When placement is submuscular, the force of the muscle can shift the position of the implant before there is enough healing to hold it in place. For this reason, submuscular implants may seem abnormally high for a period of time, although eventually they usually settle to a desirable position. If they don’t (and this occurs in about 3 to 5% of patients with submuscular placement), an endoscopic procedure similar to that used for treatment of capsular contracture is effective at allowing proper positioning of the implant. While this procedure is almost alway successful, it does represent a possible added expense. For that reason, we are seeing more and more patients choose the subglandular approach.
We offer the choice of placement to our patients. Currently most are still electing to have their implants placed beneath the muscle. If we feel there is a significant advantage to placement above or below, we will discuss it with you, but in many cases, it is simply personal preference.
INCISIONS
There are several ways in which the implant can be inserted. An incision can be made under the breast (inframammary), under the arm (transaxillary), or around the bottom of the areola (periareolar). The incision under the breast is the most common, however it is our least favorite. We have several objections to this incision. First of all, it is the area that most people would relate to breast enlargement surgery. More importantly, this approach places the scar very close to the implant. If the incision should get infected, it appears more likely that the infection would reach the implant. Finally, although inframammary scars usually heal well, we have seen some instances of scar thickening that were very difficult to improve.
Our preference with saline implants is to use a small incision made in the axilla (armpit). This keeps the incision far from the implant, places the scar in a well hidden site, and allows the operation to be performed endoscopically.
ENDOSCOPIC TRANSAXILLARY BREAST AUGMENTATION
Since blood around the implant can organize and eventually lead to thickened scar tissue, it is important to perform the surgery in as bloodless a field as possible. The use of endoscopic surgery has allowed us to carry out breast augmentation with more precision and less bleeding. Special instruments designed for this purpose allow us to work through very small incisions, monitoring the operation on a video screen. The dissection is done under close observation using endoscopic telescopes with built in video cameras. The pocket is tailored under direct vision — a great improvement over the more traditional transaxillary approach that was essentially a blind dissection.
The operation is performed under general anesthesia. A layer of elastic tape is applied after surgery and is left in place for 7 – 10 days to help support the breast. An elastic band is also applied to put downward pressure on the breast and is used for a variable period of time until the implants are properly settled.
Recovery is quite brief and patients usually return to light activity with in a day or so. Full activity is resumed within a few days. Although the breasts usually look good almost immediately after surgery, there is an improvement in the shape over the following several months.
Breast enlargement has been popular for many years. Now, with the improvements in technique and implant technology, many of the problems of the past seem to have been largely resolved. In spite of continuing controversy, all of the major studies seem to confirm the overall safety of breast implants. It is easy to understand why this operation has remained such a popular cosmetic operation for women.
It must be understood that breast augmentation alone is not a solution for women who have significant sagging of the breasts. In these cases, a combination of augmentation with breast lifting or breast reduction is the best solution.
POLYURETHANE COVERED IMPLANTS
A type of implant with a polyurethane cover was developed about thirty years ago that greatly decreased the chances of firmness. We had extensive experience with this implant and like others, found a remarkable decrease in post operative breast firmness. Unfortunately, this implant was removed from the market because of unproved suspicion that the polyurethane could break down to release a chemical that was shown to be dangerous in laboratory animals in large concentrations. Subsequent studies have shown the polyurethane to be safe. For patients who already have this implant, it is important to recognize that no human studies have yet shown any risk from the polyurethane. Based on present knowledge, it is not considered advisable for patients who have had these implants to consider removing or changing them in the absence of medical problems. The current opinion of the FDA based on thorough scientific study is that the polyurethane encasing these implants poses no measurable cancer threat.
ADDITIONAL INFORMATION
Because the status of breast implants continues to change, patients considering this operation at our Center will be provided with updated information such as material from the FDA, The Institute of Medicine, or information provided by the implant manufacturers at the time of consultation. Prospective patients should read this information carefully before electing to have surgery.
Reprints of articles written by Dr. Tobin which have appeared in the medical literature describing the endoscopic techniques described above are available from the Center on request.